The systematic names and symbols for elements of atomic numbers greater than 103 are the only approved names and symbols for those elements until the approval of trivial names by IUPAC. Nomenclature of Elements of Atomic Numbers greater than 100. The name is derived directly from the atomic number of the element using the following numerical. Atomic Number of Elements in Periodic Table. We remember from our school chemistry course that every element has its own specific atomic number. It is the same as the number of protons that the atom of each element has, so sometimes atomic number is called proton number. It is always the whole number and it ranges from 1 to 118, according to. Atomic Number of Elements in Periodic Table. We remember from our school chemistry course that every element has its own specific atomic number.It is the same as the number of protons that the atom of each element has, so sometimes atomic number is called proton number.It is always the whole number and it ranges from 1 to 118, according to the number of the element in the Periodic Table. Atomic Number 100. Atomic Number 100 is belong to element of Fermium. Chemical symbol for Fermium is Fm. Number of protons in Fermium is 100. Atomic weight of Fermium is 257 u or g/mol.
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
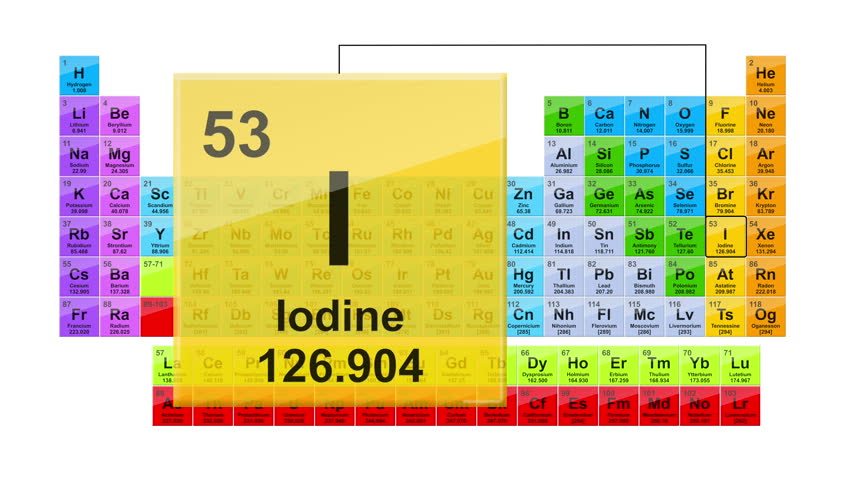
Periodic Table Of Elements - Atomic Number, Atomic Mass ..

For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
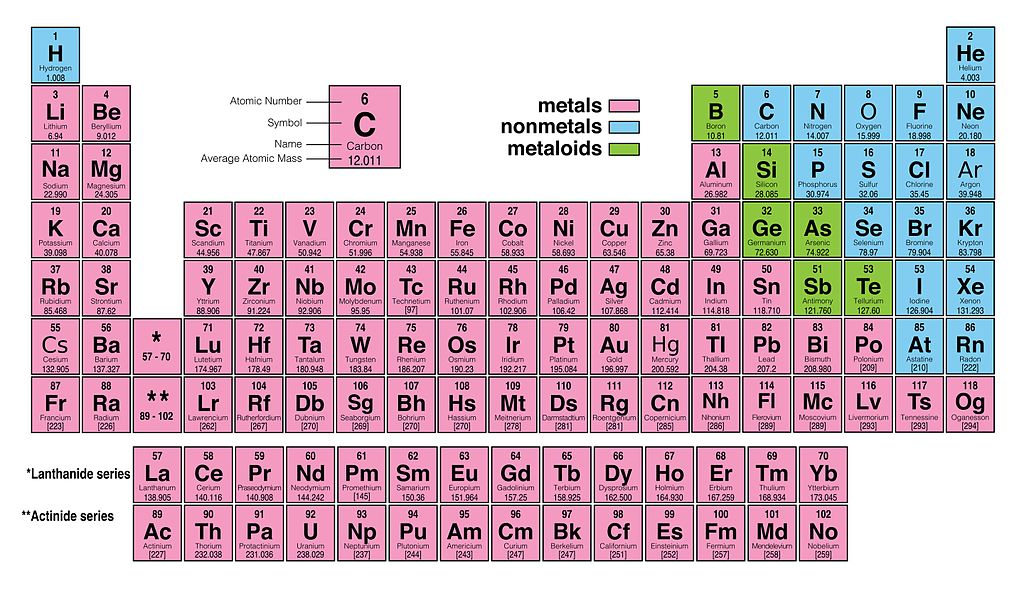
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
Stream live and catch up TV from your Sky package on your phone, tablet or laptop. Included with Sky TV at no extra cost Watch all around the home and on the move Download on up to six devices.
Commission on the Nomenclature of Inorganic Chemistry(Rules Approved 1978)
https://www.qmul.ac.uk/sbcs/iupac/AtWt/element.htmlWorld Wide Web version Prepared by G. P. Moss
School of Biological and Chemical Sciences, Queen Mary and Westfield College,
Mile End Road, London, E1 4NS, UK
g.p.moss@qmul.ac.uk
These Rules are as close as possible to the published version prepared by J. Chatt [see Pure Appl. Chem., 1979, 51, 381-384. Copyright IUPAC, reproduced with the permission of IUPAC]. If you need to cite these rules please quote these references as their source.
A PDF of the document is available.

Elements of atomic numbers of 101 to 103 have trivial names and corresponding two letter symbols approved by IUPAC. The status of these names and symbols is in no way affected by the recommendation of systematic names for elements of atomic numbers greater than 100.
Scrivener supports Markdown in that it allows you to easily structure your document with chapters and sections while letting you to use Markdown (actually MultiMarkdown) to format your document.

Elements of atomic numbers greater than 103 are often referred to in the scientific literature but receive names only after they have been 'discovered'. Names are needed for indexing and other purposes and the Commission on Nomenclature of Inorganic Chemistry was asked to make recommendations concerning names and symbols of the heavy 'unknown' elements. The Commission decided that these elements would be best named systematically and that names should accord with the following principles:
(i) The names should be short and obviously related to the atomic numbers of the elements.
(ii) The names should end in 'ium' whether the element was expected to be a metal or otherwise.
(iii) The symbols for the systematically named elements should consist of three letters.
(iv) The symbols should be derived directly from the atomic numbers and be visually related to the names as far as possible.
The reasons for principles (i), (ii), and (iv) are obvious but those for (iii) are not so immediately apparent. The Commission recommends the use of three-letter symbols because any systematically derived set of two-letter symbols will tend to duplicate some of the two-letter symbols of elements of atomic numbers less than 104. Any ad hoc method of removing such duplication will destroy the systematic derivation of the symbol.
The existence of a systematic nomenclature for the unknown elements does not deny the right of 'discoverers' of new elements to suggest other names to the Commission after their discovery has been established beyond all doubt in the general scientific community. For elements 101-103 the systematic names are minor alternatives to the trivial names already approved by IUPAC. The systematic names and symbols for elements of atomic numbers greater than 103 are the only approved names and symbols for those elements until the approval of trivial names by IUPAC.
The systematic names and symbols for elements of atomic numbers greater than 103 are the only approved names and symbols for those elements until the approval of trivial names by IUPAC. Nomenclature of Elements of Atomic Numbers greater than 100. The name is derived directly from the atomic number of the element using the following numerical. Atomic Number of Elements in Periodic Table. We remember from our school chemistry course that every element has its own specific atomic number. It is the same as the number of protons that the atom of each element has, so sometimes atomic number is called proton number. It is always the whole number and it ranges from 1 to 118, according to. Atomic Number of Elements in Periodic Table. We remember from our school chemistry course that every element has its own specific atomic number.It is the same as the number of protons that the atom of each element has, so sometimes atomic number is called proton number.It is always the whole number and it ranges from 1 to 118, according to the number of the element in the Periodic Table. Atomic Number 100. Atomic Number 100 is belong to element of Fermium. Chemical symbol for Fermium is Fm. Number of protons in Fermium is 100. Atomic weight of Fermium is 257 u or g/mol.
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
Periodic Table Of Elements - Atomic Number, Atomic Mass ..
For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
Stream live and catch up TV from your Sky package on your phone, tablet or laptop. Included with Sky TV at no extra cost Watch all around the home and on the move Download on up to six devices.
Commission on the Nomenclature of Inorganic Chemistry(Rules Approved 1978)
https://www.qmul.ac.uk/sbcs/iupac/AtWt/element.htmlWorld Wide Web version Prepared by G. P. Moss
School of Biological and Chemical Sciences, Queen Mary and Westfield College,
Mile End Road, London, E1 4NS, UK
g.p.moss@qmul.ac.uk
These Rules are as close as possible to the published version prepared by J. Chatt [see Pure Appl. Chem., 1979, 51, 381-384. Copyright IUPAC, reproduced with the permission of IUPAC]. If you need to cite these rules please quote these references as their source.
A PDF of the document is available.
Recommendations for the Naming of Elements of Atomic Numbers Greater than 100Elements of atomic numbers of 101 to 103 have trivial names and corresponding two letter symbols approved by IUPAC. The status of these names and symbols is in no way affected by the recommendation of systematic names for elements of atomic numbers greater than 100.
Scrivener supports Markdown in that it allows you to easily structure your document with chapters and sections while letting you to use Markdown (actually MultiMarkdown) to format your document. Click on the Compile button to turn your MultiMarkDown text into HTML. Make sure the pull-down menu option for Format As is Original. Select in Compile For pull-down menu the option Multimarkdown - HTML. The settings for Scrivener to turn MultiMarkDown into HTML. Jun 02, 2018 In a simple Scrivener project consisting of one folder, the Markdown output uses the folder name as a heading, and the folder text as the body text after the heading. Sighted users drag and drop binder documents to control order and nesting level. This is accessible with VoiceOver using control+command plus any one of the arrow keys.
Elements of atomic numbers greater than 103 are often referred to in the scientific literature but receive names only after they have been 'discovered'. Names are needed for indexing and other purposes and the Commission on Nomenclature of Inorganic Chemistry was asked to make recommendations concerning names and symbols of the heavy 'unknown' elements. The Commission decided that these elements would be best named systematically and that names should accord with the following principles:
(i) The names should be short and obviously related to the atomic numbers of the elements.
(ii) The names should end in 'ium' whether the element was expected to be a metal or otherwise.
(iii) The symbols for the systematically named elements should consist of three letters.
(iv) The symbols should be derived directly from the atomic numbers and be visually related to the names as far as possible.
The reasons for principles (i), (ii), and (iv) are obvious but those for (iii) are not so immediately apparent. The Commission recommends the use of three-letter symbols because any systematically derived set of two-letter symbols will tend to duplicate some of the two-letter symbols of elements of atomic numbers less than 104. Any ad hoc method of removing such duplication will destroy the systematic derivation of the symbol.
The existence of a systematic nomenclature for the unknown elements does not deny the right of 'discoverers' of new elements to suggest other names to the Commission after their discovery has been established beyond all doubt in the general scientific community. For elements 101-103 the systematic names are minor alternatives to the trivial names already approved by IUPAC. The systematic names and symbols for elements of atomic numbers greater than 103 are the only approved names and symbols for those elements until the approval of trivial names by IUPAC.
Nomenclature of Elements of Atomic Numbers greater than 100
1. The name is derived directly from the atomic number of the element using the following numerical roots:
| 0 = nil | 3 = tri | 6 = hex | 9 = enn |
| 1 = un | 4 = quad | 7 = sept | |
| 2 = bi | 5 = pent | 8 = oct |
What Is The Atomic Number And Mass Number?The Number Of Protons And The Number Of Neutrons Shall Determine The Mass Number Of An Element. Since The Isotopes Of An Element Have Slightly Diff..
3. The symbol of the element is composed of the initial letters of the numerical roots which make up the name.
4. The root 'un' is pronounced with a long 'u', to rhyme with 'moon'. In the element names each root is to be pronounced separately.
| Atomic number | Name | Symbol |
| 101 | Mendelevium (Unnilunium) | Md* |
| 102 | Nobelium (Unnilbium) | No* |
| 103 | Lawrencium (Unniltrium) | Lr* |
| 104 | Unnilquadium | Unq |
| 105 | Unnilpentium | Unp |
| 106 | Unnilhexium | Unh |
| 107 | Unnilseptium | Uns |
| 108 | Unniloctium | Uno |
| 109 | Unnilennium | Une |
| 110 | Ununnilium | Uun |
| 111 | Unununium | Uuu |
| 112 | Ununbium | Uub |
| 113 | Ununtrium | Uut |
| 114 | Ununquadium | Uuq |
| 115 | Ununpentium | Uup |
| 116 | Ununhexium | Uuh |
| 117 | Ununseptium | Uus |
| 118 | Ununoctium | Uuo |
| 119 | Ununennium | Uue |
| 120 | Unbinilium | Ubn |
| 121 | Unbiunium | Ubu |
| 130 | Untrinilium | Utn |
| 140 | Unquadnilium | Uqn |
| 150 | Unpentnilium | Upn |
| 160 | Unhexnilium | Uhn |
| 170 | Unseptnilium | Usn |
| 180 | Unoctnilium | Uon |
| 190 | Unennilium | Uen |
| 200 | Binilnilium | Bnn |
| 201 | Binilunium | Bnu |
| 202 | Binilbium | Bnb |
| 300 | Trinilnilium | Tnn |
| 400 | Quadnilnilium | Qnn |
| 500 | Pentnilnilium | Pnn |
| 900 | Ennilnilium | Enn |
Atomic Number 100 Iupac Name
* To correspond to the systematic names, the systematic symbols would be Unu, Unb and Unt respectively. Return to Atomic weight tableReturn to Periodic table
Return to IUPAC Chemical Nomenclature home page
