- Iodine Valence Electrons Lewis Dot Structure
- List Of Valence Electrons For Each Element
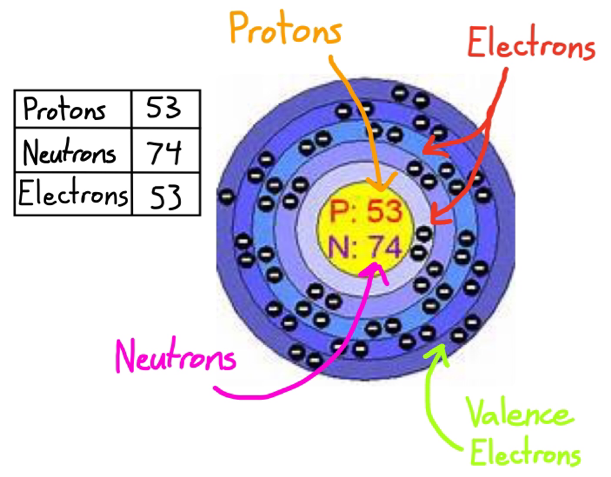
- Iodine Valence Electrons
Iodine has seven valence electrons. A valence electron is an electron in the outer shell of an atom that is able to participate in bonding. While iodine has 53 electrons, all but seven are in the inner shells of the iodine atom. In chemistry, an atom has a full shell if it has eight valence electrons. Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
The octet rule is so simple and useful in introducing the basic concepts of bonding that you might assume that it is always obeyed. In Section 8.2, however, we noted its limitation in dealing with ionic compounds of the transition metals. The octet rule also fails in many situations involving covalent bonding. These exceptions to the octet rule are of three main types:
1. Molecules with an odd number of electrons;
2. Molecules in which an atom has less than an octet;
3. Molecules in which an atom has more than an octet.
Odd Number of Electrons
In the vast majority of molecules, the number of electrons is even, and complete pairing of electrons occurs. However, in a few molecules such as ClO2, NO, and NO2, the number of electrons is odd. For example, NO contains 5 + 6 = 11 valence electrons. Obviously, complete pairing of these electrons is impossible, and an octet around each atom cannot be achieved.
Less than an Octet
A second type of exception occurs when there are fewer than eight electrons around an atom in a molecule or ion. This is also a relatively rare situation and is most often encountered in compounds of boron and beryllium. For example, let's consider boron trifluoride, BF3. If we follow the first four steps of the procedure at the beginning of Section 8.6 for drawing Lewis structures, we have the following structure:
We see that there are only six electrons around the boron atom. We could complete the octet around boron by forming a double bond (Step 5). In so doing, we see that there are three equivalent resonance structures:

However, in forming these Lewis structures, we have forced a fluorine atom to share additional electrons with the boron atom. This is inconsistent with the high electronegativity of fluorine. We don't expect the fluorine atoms to share additional electrons with the boron atom. (When there is a double bond between B and F, the F atom has a formal charge of +1, and the less electronegative B atom has a formal charge of -1, an unfavorable situation.) We conclude that the Lewis structures in which there is a BF double bond are less important than the one in which there is less than an octet around boron:
We usually represent BF3 solely by the leftmost resonance structure in which there are only six electrons around boron. The chemical behavior of BF3 is consistent with this representation. Thus, BF3 reacts very energetically with molecules having an unshared pair of electrons that can be used to form a bond with boron. For example, it reacts with ammonia, NH3, to form the compound NH3BF3:
In this stable compound, boron has an octet of electrons.
More than an Octet
The third and largest class of exceptions consists of molecules or ions in which there are more than eight electrons in the valence shell of an atom. As an example, consider PCl5. When we draw the Lewis structure for this molecule, we are forced to 'expand' the valence shell and place 10 electrons around the central phosphorus atom:
Other examples of molecules and ions with 'expanded' valence shells are SF4, AsF6-, and ICl4-. The corresponding molecules with a second-period atom, such as NCl5 and OF4, do not exist. Let's take a look at why expanded valence shells are observed only for elements in period 3 and beyond in the periodic table.

Elements of the second period have only the 2s and 2p valence orbitals available for bonding. Because these orbitals can hold a maximum of eight electrons, we never find more than an octet of electrons around elements from the second period. We can extend this idea of available valence orbitals to provide an appealing explanation for the presence of expanded valence shells in the third period and beyond. In addition to ns and np orbitals, elements from the third period and beyond have unfilled nd orbitals that can be used in bonding. For example, the orbital diagram for the valence shell of a phosphorus atom is as follows:
Although third-period elements such as phosphorus often satisfy the octet rule, as in PCl3, they also often exceed an octet by seeming to use their empty d orbitals to accommodate additional electrons. (On the basis of recent theoretical calculations, some chemists have questioned whether valence d orbitals are actually used in the bonding of molecules with expanded valence shells. Nevertheless, the presence of valence d orbitals in period 3 and below provides the simplest explanation of this phenomenon, especially within the scope of a general chemistry textbook.)
Size also plays an important role in determining whether an atom can accommodate more than eight electrons. The larger the central atom, the larger the number of atoms that can surround it. The occurrences of expanded valence shells therefore increase with increasing size of the central atom. The size of the surrounding atoms is also important. Expanded valence shells occur most often when the central atom is bonded to the smallest and most electronegative atoms, such as F, Cl, and O.
Sample Exercise 8.12
Draw the Lewis structure for ICl4-.
SOLUTION Iodine (group 7A) has 7 valence electrons; each chlorine (group 7A) also has 7; an extra electron is added to account for the 1- charge of the ion. Therefore, the total number of valence electrons is 7 + 4(7) + 1 = 36. The I atom is the central atom in the ion. Putting 8 electrons around each Cl atom (including a pair of electrons between I and each Cl to represent the single bonds between these atoms) requires 8 × 4 = 32 electrons. We are thus left with 36- 32 = 4 electrons to be placed on the larger iodine:
Iodine has 12 electrons around it, exceeding the common octet of electrons.

However, in forming these Lewis structures, we have forced a fluorine atom to share additional electrons with the boron atom. This is inconsistent with the high electronegativity of fluorine. We don't expect the fluorine atoms to share additional electrons with the boron atom. (When there is a double bond between B and F, the F atom has a formal charge of +1, and the less electronegative B atom has a formal charge of -1, an unfavorable situation.) We conclude that the Lewis structures in which there is a BF double bond are less important than the one in which there is less than an octet around boron:
We usually represent BF3 solely by the leftmost resonance structure in which there are only six electrons around boron. The chemical behavior of BF3 is consistent with this representation. Thus, BF3 reacts very energetically with molecules having an unshared pair of electrons that can be used to form a bond with boron. For example, it reacts with ammonia, NH3, to form the compound NH3BF3:
In this stable compound, boron has an octet of electrons.
More than an Octet
The third and largest class of exceptions consists of molecules or ions in which there are more than eight electrons in the valence shell of an atom. As an example, consider PCl5. When we draw the Lewis structure for this molecule, we are forced to 'expand' the valence shell and place 10 electrons around the central phosphorus atom:
Other examples of molecules and ions with 'expanded' valence shells are SF4, AsF6-, and ICl4-. The corresponding molecules with a second-period atom, such as NCl5 and OF4, do not exist. Let's take a look at why expanded valence shells are observed only for elements in period 3 and beyond in the periodic table.
Elements of the second period have only the 2s and 2p valence orbitals available for bonding. Because these orbitals can hold a maximum of eight electrons, we never find more than an octet of electrons around elements from the second period. We can extend this idea of available valence orbitals to provide an appealing explanation for the presence of expanded valence shells in the third period and beyond. In addition to ns and np orbitals, elements from the third period and beyond have unfilled nd orbitals that can be used in bonding. For example, the orbital diagram for the valence shell of a phosphorus atom is as follows:
Although third-period elements such as phosphorus often satisfy the octet rule, as in PCl3, they also often exceed an octet by seeming to use their empty d orbitals to accommodate additional electrons. (On the basis of recent theoretical calculations, some chemists have questioned whether valence d orbitals are actually used in the bonding of molecules with expanded valence shells. Nevertheless, the presence of valence d orbitals in period 3 and below provides the simplest explanation of this phenomenon, especially within the scope of a general chemistry textbook.)
Size also plays an important role in determining whether an atom can accommodate more than eight electrons. The larger the central atom, the larger the number of atoms that can surround it. The occurrences of expanded valence shells therefore increase with increasing size of the central atom. The size of the surrounding atoms is also important. Expanded valence shells occur most often when the central atom is bonded to the smallest and most electronegative atoms, such as F, Cl, and O.
Sample Exercise 8.12
Draw the Lewis structure for ICl4-.
SOLUTION Iodine (group 7A) has 7 valence electrons; each chlorine (group 7A) also has 7; an extra electron is added to account for the 1- charge of the ion. Therefore, the total number of valence electrons is 7 + 4(7) + 1 = 36. The I atom is the central atom in the ion. Putting 8 electrons around each Cl atom (including a pair of electrons between I and each Cl to represent the single bonds between these atoms) requires 8 × 4 = 32 electrons. We are thus left with 36- 32 = 4 electrons to be placed on the larger iodine:
Iodine has 12 electrons around it, exceeding the common octet of electrons.
Practice Exercise
Iodine Valence Electrons Lewis Dot Structure
Which of the following atoms is never found with more than an octet of electrons around it: S, C, P, Br? Answer: C
List Of Valence Electrons For Each Element
At times you may see Lewis structures written with expanded octets even though structures can be written with an octet. For example, consider the following Lewis structures for the phosphate ion, PO43-:
Iodine Valence Electrons
The formal charges on the atoms are shown in red. On the left the P atom has an octet; on the right the P atom has an expanded octet of five electron pairs. The structure on the right is often used for PO43- because it has smaller formal charges on the atoms. The best representation of PO43- is a series of Lewis structures in resonance with one another. However, recent theoretical calculations, based on quantum mechanics, suggest that the structure on the left is the best single Lewis structure for the phosphate ion. In general, when choosing between alternative Lewis structures, you should choose one that satisfies the octet rule if it is possible to do so.
